| |||||||
|
|
|||||||
|
|
|||||||
| Pressure solution and Clay Content | |||||||
|
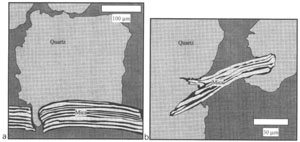
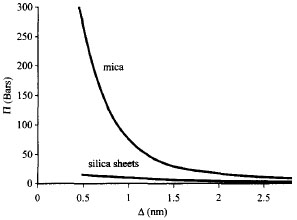
Pressure solution is generally enhanced at the contact with clay minerals, presumably because these minerals increase the effective grain contact and thickness of the adsorbed water film, which in turn better host diffusion. It is well known that a grain of quartz in contact with a clay flake dissolves faster than when in contact with another grain of quartz (Heald, 1955; Bjorkum, 1996). In sandstones, micas can penetrate quartz and the flat contact between the two minerals is the result of pressure solution. It is observed that quartz dissolves at the contact with mica, whose surface seems to be unaffected by the pressure solution (Figure 1). In a case in which diffusion is the slower step of a pressure solution process, as in pressure solution at great depths, the thickness of water film, which is affected by the grain mineralogy, becomes an important factor. The thickness of water film has been measured or calculated for many minerals and varies from a few angstroms to several nanometers (Heidug, 1995). In Figure 2, the thickness of the water film is given as a function of the disjoining pressure, i.e., the amount by which the fluid pressure acting on the fluid-solid interface exceeds the hydrostatic pressure in the bulk fluid, equivalent to effective stress, for a silica-mica system. The curves clearly indicate that the water film thickness is smaller between two sheets of silica than between two platelets of mica. Assuming that between a mica and a grain of quartz, the water film is similar to that between two micas, it is expected that the the quartz-mica contact would dictate a faster diffusion, and thus promote pressure solution. However, when clay forms coatings on the pore surface, it may retard pressure solution (Rendard et al., 1997). In this case, mineral precipitation on the pore surface is hindered because of the loss of available precipitation area. The pore fluid may quickly becomes supersaturated with dissolute so that the concentration gradient disappears and pressure solution stops. | |||||||
| Reference: |
|||||||
| Bjorkum, P.A., 1996 Heald, M.T., 1955 Heidug, W.K., 1995 Renard, F., Ortoleva, P., Gratier, J.P., 1997 |
|||||||
|
Readme | About Us | Acknowledgement | How to Cite | Terms of Use | Ⓒ Rock Fracture Knowledgebase |
|||||||