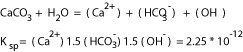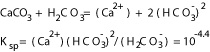| |||||||
|
|
|||||||
|
|
|||||||
| Pressure Solution and Pore Fluid Chemistry | |||||||
|
The chemistry of the pore fluid, such as the amount of carbon dioxide, alkalinty, and salinity, could alter the rate of dissolution of carbonate rocks. Unreactive pore fluid such as liquid hydrocarbon prevents pressure solution in the laboratory and in the field and inhibits precipitation. Without the presence of carbon dioxide and with the presence of only calcite, the solubility of calcite is governed by the equation in Figure 1, taking into account the hydrolysis of the carbonate anion at 25 degrees C (Krauskopf and Bird, 1995). The Calcium concentration estimated by the ion concentration product in Equation 1 is then 1.3*10(-4) M, or 5.2 mg per L. With contact with carbon dioxide, calcite solubility can be expressed in the equation in Figure 2. The solubility of calcite in water in contact with 0.1 atmosphere of carbon dioxide can be calculated as in Equation 2, producing a calcite solubility of 3.2*10(-3) M, or 128 mg per L Calcium at 25 degrees C.
Other acids, such as sulfuric from the oxidation of sulfide minerals, can also significantly increase the solubility. When the fluid in the pressure solution originally contains Calcium, the amount of Calcite that can dissolve in it decreases accordingly. The presence of other ions in the solution may also shield the Calcium and carbonate ions from interacting and precipitating, represented by ionic strength (Faure, 1998). | |||||||
| Reference: |
|||||||
| Faure, G., 1998 Krauskopf, K.B., Bird, D.K., 1995 |
|||||||
|
Readme | About Us | Acknowledgement | How to Cite | Terms of Use | Ⓒ Rock Fracture Knowledgebase |
|||||||