| ||||||||||||
|
|
||||||||||||
|
|
||||||||||||
| Solubility of Minerals and Rocks | ||||||||||||
|
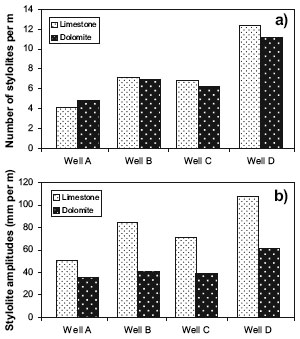
Solubility is the susceptibility of a rock to be dissolved; and it is the most important factor that effects whether pressure solution can occur. Pressure solution is common in carbonates and siliciclastics, but have also been found in rhyolite, pegmatite, chert, and granite. The solubility of a particular rock is dependent on the mineral composition of the rock, temperature, pressure, and the fluid chemistry. Listed below are minerals with relative solubilities from the highest to the lowest (from Trurnit, 1968). (1) halite and potassium salts; (2) calcite; (3) dolomite; (4) anhydrite; (5) gypsum; (6) amphibolite and pyroxene; (7) chert; (8) quartzite; (9) quartz, glauconite, rutile, and hematite; (10) feldspars and cassiterite; (11) mica and clay minerals; (12) arsenopyrite; (13) tourmaline and sphene; (14) pyrite; (15) zircon; (16) chromite. Of course, other factors may alter this order. Many authors studied the solubility of carbonates. Zhang et al (2002) showed that increased magnesium reduces the rate of pressure solution. Peacock and Azzam (2006) reported twice as much pressure solution in limestones as in dolomites from core in the Khuff Formation of UpperPermian and Lower Triassic age in Abu Dhabi. They found similar numbers of stylolites per meter occur in the limestones and the dolomites (Figure 1a), but cumulative stylolite amplitudes (Figure 1b) and insoluble residue thicknesses per meter are about twice as much as in limestone as in dolomites. Average stylolite amplitudes are about 10 mm in limestones and 6 mm in dolomites. The difference in amplitude indicats the rate of pressure solution in limestones is twice that in dolomites under mechanical conditions in the Khuff Formation, or that the limestones have been affected by pressure solution for twice as long as the dolomites. Since there are no apparent difference in the textures of dolomites and limestones that may be responsible for the difference in the amount of pressure solution, it is inferred that the rate of pressure solutions in limestone is roughly twice of that of dolomite. The followings are examples of carbonates, including calcite, limestone and dolomite plus tufa: The two major types of carbonate rocks are limestone and dolomite, composed primarily the mineral of calcite (CaCO3) and dolomite (CaMg(CO3)2) respectively. Chalk and tufa are also sedimentary rocks of carbonate types. Marble is the metamorphic carbonate rock. Compared to other common rocks, carbonates, especially limestones, exhibit an unusual characteristic; they can dissolved and precipitated easily. Under stress-free condition, erosional landforms form; when pressure is high either due to either overburden gravity or tectonic loading, pressure solution seams form. Most carbonate rocks originate as sedimentary deposits in marine environments. During lithification, compaction, cementation, and dolomitization may act on the deposits and greatly change their porosity and permeability. However, the principal postdepositional change in carbonate rocks is the dissolution of part of the rock by circulating, slightly acidic ground water. Solution openings in carbonate rocks range from small tubes and widened joints to caverns that may be tens of meters wide and hundreds to thousands of meters in length. When these solution openings form connected networks, large amounts of water or oil and gas may be stored and produced, although the undissolved rock between the large openings may be almost impermeable. If carbonate rocks are buried further down or subjected to tectonic deformation, pressure solution is likely to take place. Pressure solution reduces pore space by dissolution and precipitation as cement. The resulted pressure solution seams may have permeability much lower than the surrounding rocks, thus can compartment aquifers or reservoirs and have strong effect on fluid fluid. Limestone (Figure 2) is a sedimentary rock composed largely of the mineral calcite (calcium carbonate: CaCO3). Limestone often contains variable amounts of silica in the form of chert or flint, as well as varying amounts of clay, silt and sand as dissemination, nodules, or layers within the rock. The primary source of the calcite in limestone is of primarily marine organisms (Figure 3).
Calcite is partially soluble, forming pressure solution seams under pressure and erosional landforms like pot holes (Figure 4), caves, and gorges on the earth surface chemical dissolution. Calcite exhibits an unusual characteristic called retrograde solubility in which it becomes less soluble in water as the temperature increases.
Limestone is especially popular in architecture, and many landmarks around the world, especially in North America and Europe, are made of this rock. Dolomite is a particular carbonate rock (Figure 1), composed of primarily calcium magnesium carbonate CaMg(CO3)2. Dolomite rock (also called dolostone) is composed predominantly of the mineral dolomite. Limestone that is partially replaced by dolomite is referred to as dolomitic limestone, or in old U.S. geologic literature as magnesian limestone. Vast amount of occurrence is present in the geological record, but it is relatively rare in modern environments. Mineral dolomite has physical properties similar to those of the mineral calcite. However, it does not rapidly and easily dissolve in dilute hydrochloric acid. Zhang et al (2002) reported that increased magnesium appears to reduce the rate of pressure solution. Tufa is a rough, thick calcium carbonate deposit that forms by precipitation from bodies of water with a high dissolved calcium content. | ||||||||||||
| Reference: |
||||||||||||
| Peacock, D.C.P., Azzam, I. N., 2006 Trurnit, P., 1968 Zhang, X.D., Salemans, J., Peach, C.J., Spiers, C.J., 2002 |
||||||||||||
|
Readme | About Us | Acknowledgement | How to Cite | Terms of Use | Ⓒ Rock Fracture Knowledgebase |
||||||||||||