| |||||||
|
|
|||||||
|
|
|||||||
| Pressure Solution and the Role of Water | |||||||
|
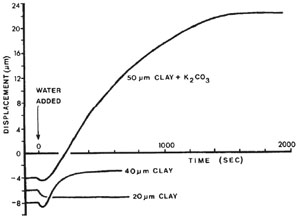
A crucial parameter for pressure solution is the presence of water in the pores because it is the medium of transport. Rutter (1983) was the first to demonstrate by experiments that solid-state grain-boundary diffusion along grain contact is too slow a process to allow significant strain to occur at geological strain rates. Rutter's experiments are done by first compacting thin kaolinite film in dry conditions under 100 MPa. Kaolinite is a non-swelling clay, which means its lattice parameters do not change on wetting and it does not dissolve in water. After a few minutes, when zero compaction rate is achieved, a drop of water is added to the edge of the film. The results of the experiments are shown in Figure 1. Typical behavior of 20 μm kaolinite film shows expansion only. 40 μm kaolinite film shows initial expansion followed by further compaction, presumably due to the facilitation of sliding between particles by the adsorbed water. The results on pure kaolinite film suggest that water film can exist in stressed mineral interfaces, over a stress range encompassing a substantial part of that likely to be encountered during natural rock deformation. Experiments are also made with 50 μm thick film of potassium carbonate and kaolinite mixture. Potassium carbonate is easily soluble. The addition of a drop of water causes compaction to begin immediately, sometimes preceded by a small expansion (Figure 1). The compaction rate decayed steadily with time, becoming zero after several thousand seconds, when it was inferred that the soluble salt had been totally consumed. Compared with what is observed with pure kaolinite film, the compaction is much larger since the soluble potassium carbonate dissolves under pressure. Adding water apparently facilitates the dissolution under pressure, as the compaction immediately begins once water is added when zero compaction rate is achieved at time zero. Rutter credited the compaction associated with additional watering to a thin water film present in the mineral interface, assisting the diffusion of the dissolved mineral. He also noted that high stress and high temperature will likely reduce the thickness of the water film and degrade the transport properties of the remaining film. Based on the mobility of pore fluid, a pressure solution system can be classified as a closed system where pore fluid is locally confined, or an open system where pore space is connected and pore fluid can be brought out of the system. In a closed system, since pore fluid is confined locally, dissolution, diffusion, and re-crystallization will be localized in the pores where fluid is present. This effect can create compacted regions in the sediment. The porosity of other regions stay the same. Shortening, reduction in porosity, and permeability is expected to be limited for closed systems. In open systems, dissolved mineral is carried out of the system through the connected pore space. Thus, diffusion is no longer a factor affecting the strain rate of the pressure solution, but the rate of fluid flow as well as the composition of the fluid do. Safaricz and Davison (2005) reported an extensive open pressure solution system in chalk at the Machar and Flamborough oil field, United Kingdom Central Graben, the North Sea. The authors estimated that 57% of the dissolved chalk had been removed from the system by over 3000 cubic meters of water. This extraordinarily large amount of fluid throughput is made possible by being at the outlet points in a fluid convection cell in the sedimentary basin. The thickness of water film is effected by mineralogy, probably because of electric surface charge, and effective stress and varies from a few angstroms to several nanometers. The thickness of the water film is given as a function of the disjoining pressure, i.e., the amount by which the fluid pressure acting on the fluid-solid interface exceeds the hydrostatic pressure in the bulk fluid, equivalent to effective stress. On one hand, under most basin conditions, the thickness of water film decreases exponentially with stress until the lower limit of 0.5 nm is reached which represents the thickness of two layers of water molecules. On the other hand, the curves clearly indicate that the water film thickness is smaller between two sheets of silica than between two micas. | |||||||
| Reference: |
|||||||
| Rutter, E.H., 1983 Safaricz, M., Davison, I., 2005 |
|||||||
|
Readme | About Us | Acknowledgement | How to Cite | Terms of Use | Ⓒ Rock Fracture Knowledgebase |
|||||||