| |||||||
|
|
|||||||
|
|
|||||||
| Residues | |||||||
|
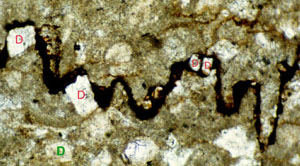
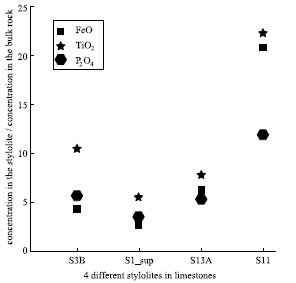
Pressure solution seams represent relatively non-diffusible and insoluble residues left behind after soluble material dissolved and diffused away. The residues are commonly dark color and made up of mostly or predominantly clay minerals, oxides, and organic matter (Figure 1). It is often inferred from mass transfer and composition analysis that the residual material accumulating within the pressure solution seams is in proportion to the volume of the diffusible rock material swept through. Stockdale (1922) was the first geologist who established the relation between the residue and the insoluble components of the host rock by studying the limestones from the United States craton, specifically, from Indiana and Tennessee, USA. He showed that the material in the residue had the same mineralogical composition, oxide ratios, and percentage of organic matter as the insoluble components of the adjacent host rock. More recently, Renard et al. (2004) and Hassan (2007) showed via X-ray fluorescence and by SEM, respectively, that residues are enriched in aluminum, silica, iron, sulfur, potassium, iron, titanium, and phosphorus compared with the bulk rock. Renard and coauthors noted that the concentration ratios between elements are not conserved (Figure 2) and provided several explanations:
1) The analyzed elements may be shared by a number of minerals that have different solubility. This is commonly associated with pressure solution seam differentiation. 2) The solution seams may have nucleated on pre-existing surfaces, such as clay seams that had a different composition from the bulk rock. 3) Fluids may have percolated along the solution seams and may have removed some dissolved elements. Please see the section, 'Thickness of Residue' for more discussion on this topic. | |||||||
| Reference: |
|||||||
| Hassan, H.M., 2007 Renard, F., Schmittbuhl, J., Gratier, J.P., Meakin, P., Merino, E., 2004 Stockdale, P.B., 1922 |
|||||||
|
Readme | About Us | Acknowledgement | How to Cite | Terms of Use | Ⓒ Rock Fracture Knowledgebase |
|||||||